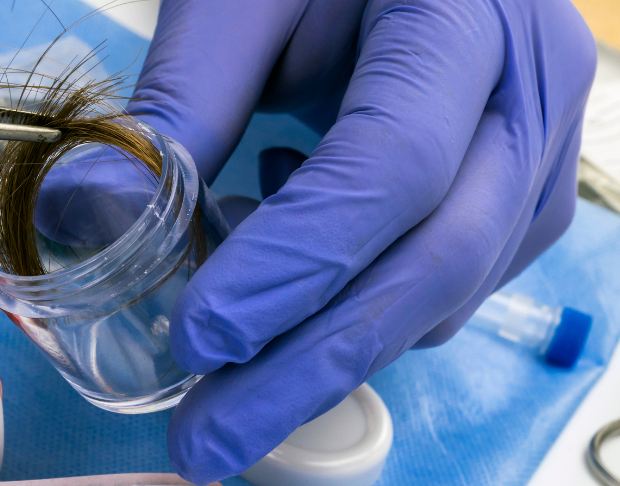
Hair tissue mineral analysis (HTMA) is an analytical test that assays the mineral composition of the hair. Hair is the second most metabolically active tissue and by the very nature of its formation as a biological sample, may provide a permanent record of metabolic activity occurring within the body during its period of growth. A mineral deficiency or excess revealed in the hair test can indicate a possible deficiency, excess, or bio-unavailability of that mineral within the body. If a patient is suffering from an illness or syndrome and the cause cannot be identified through ordinary testing procedures, or if therapy is not completely effective, hair analysis can be very helpful.
HTMA can greatly assist the clinician in assessing a patient’s health and nutritional status. Used in conjunction with other familiar diagnostic tests, HTMA can provide a more wholistic and comprehensive picture upon which h to base the most effective nutritional therapy.
Hair specimen can be collected more quickly and easily than blood, urine, or any other tissue, using a non-invasive method.
Hair analysis is more cost-effective than mineral testing through other means.
Unlike blood, hair is less susceptible to the homeostatic mechanisms that quickly affect trace element levels.
Long-term deviations of mineral retention or losses are more easily detected in hair than blood.
Concentrations of most elements in the hair are significantly higher than found in the blood and other tissues.
Hair provides a record of past as well as present trace element levels, i.e. biological activity.
Hair provides information of substances entering the hair from the blood serum as well as from external sources.
Hair is invaluable in the assessment of toxic metal levels.
The hair sample is tested in a licensed clinical laboratory with a series of chemical and high temperature methods. It is interpreted and reported by a specialized team and a special computer program.
The hair to be collected should be untreated, i.e. not permed, dyed or bleached. If the hair has been chemically treated, sampling should be delayed until sufficient new virgin growth has emerged to allow collection. The hair should be free of all gels, oils and hair creams prior to sample collection. Each collected sample should be taken in small portions from at least four to five different locations of the scalp. The recommended areas for collection are the nape of the neck, posterior vertex and posterior temporal regions.
High grade stainless steel scissors or thinning shears should be used to cut the hair as close to the scalp as possible. The length of the collected hair should not exceed 4 cm. The proximal portion (4cm closest to the root) should be retained and the excess discarded. The weight requested for a hair specimen is 125 milligrams or one full heaping teaspoon. Upon cutting the sample, the hair should be placed directly into a clean hair specimen envelope normally provided by the laboratory and then sealed with the glue flap only. Do not use plastic bags in place of the standard paper envelopes to hold the hair specimen. In addition, do not use staples, paper clips, adhesive tape, aluminum foil or other metal and paper material of any kind to seal, secure or wrap the hair envelope and/or the hair specimen contained within.
Ovital’s hair analysis is a unique aid in recovery boost planning. Thus, your doctor can design an effective nutrition program for you and boost your recovery boost.
Keats House, 24 -26
St Thomas St London, SE1 9RS, Uk
+90 850 441 6656
ovital@hushmail.com
